Glaucoma News

Glaucoma Research Foundation Announces the 2025 Shaffer Grants for Innovative Glaucoma Research
“These one-year incubator grants attract much-needed brainpower to the field of glaucoma and move us ever closer to the discovery of new treatments to preserve and restore vision.”

James Tribble, PhD receives 2025 Shaffer Research Prize from Glaucoma Research Foundation
The Shaffer Prize recognizes a researcher whose project best exemplifies the pursuit of innovative ideas in the quest to cure glaucoma.

Glaucoma Research Foundation to Host 14th Annual Glaucoma 360 Event in San Francisco
This year’s Glaucoma 360 will take place from February 6 to 8, 2025.
Bausch + Lomb and Glaucoma Research Foundation Launch Second Annual ‘Faces of Glaucoma’ Campaign and Fundraising Challenge
Throughout January, Bausch + Lomb and GRF will spotlight educational resources and share stories of individuals and families living with glaucoma to raise awareness of the disease.

Drs. Shaffer, Hetherington, and Hoskins: A Legacy of Innovation in Glaucoma Care
In 1978, three visionary heroes launched an enterprise that has gone on to change the course of glaucoma care.

Collaborative Research Team to Focus on Developing Whole Eye Transplants to Restore Vision
Vision-restoring whole eye transplants may soon become a reality, thanks to a collaborative effort led by Jeffrey Goldberg, MD, PhD, chair of ophthalmology at the Byers Eye Institute at Stanford.

Third Annual “Solving Neurodegeneration” Catalyst Meeting — Neuroscience Experts Assemble in Boston to Seek Innovative Cures
Meeting participants presented new, unpublished research results, followed by group discussions in which new ideas were encouraged.

Sixth Annual Glaucoma Patient Summit “Truly Amazing”
Patients learned about new diagnostic and treatment techniques, research updates, and how to best navigate and advocate in their glaucoma care.

Glaucoma 360: An Incubator for Innovation
Glaucoma 360 is a series of three days of annual events — hosted by Glaucoma Research Foundation — aiming to prevent vision loss from glaucoma and speed the cure.
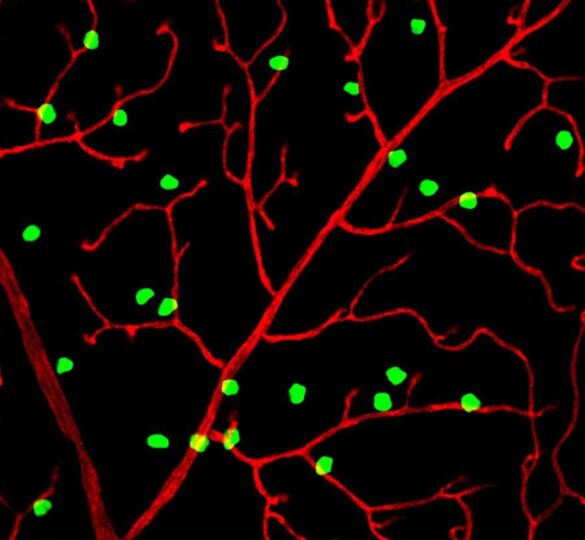
New Research from GRF-funded Investigators Reports Discovery of New Type of Neuron in the Eye
The discovery of how intricate networks of blood vessels in the eye and brain are formed could inspire new treatments for glaucoma, diabetic retinopathy, and stroke.

David S. Friedman, MD, PhD, MPH joins Glaucoma Research Foundation Board of Directors
“David Friedman is a distinguished ophthalmologist and a leader in glaucoma clinical care, research, and education,” said Thomas Brunner, President and CEO, Glaucoma Research Foundation.

Award-winning author Kristin Smedley will deliver Keynote at Glaucoma Research Foundation 2024 Patient Summit
The sixth annual Patient Summit will bring together patients, caregivers, and vision experts for inspiration, information, and insight on living with glaucoma.

GRF Honors Optics Pioneer, Charles Munnerlyn, PhD, and Judith Munnerlyn at Annual Gala
Judy and Charles Munnerlyn were honored to recognize their extraordinary contributions to improving vision for people around the world.

Glaucoma Research Foundation establishes Strategic Advisory Council
The prestigious group brings together key industry leaders and members of the corporate community dedicated to glaucoma patient care.

Tracy M. Valorie joins Glaucoma Research Foundation Board of Directors
Glaucoma Research Foundation has elected Tracy M. Valorie, BS, MBA to its Board of Directors.

Glaucoma Research Foundation Awards 2024 Shaffer Prize to Myoungsup Sim, PhD
“Being recognized by the Shaffer Prize has validated the significance of my research project and its potential to advance our understanding of glaucoma,” Dr. Sim said.

Glaucoma Research Foundation Releases Informational Audiobook for Glaucoma Patients in Collaboration with Braille Institute of America
The free glaucoma audiobook, narrated by Bianca Beach, is available for patients and their families to listen, download, and share.

Glaucoma Research Foundation Announces $2.5 Million in Grants to Cure Glaucoma
“This is the largest annual research budget in our 46-year history,” said Thomas M. Brunner, GRF President and CEO.

World Glaucoma Week
The Glaucoma Research Foundation in San Francisco joins eye health organizations and eye care professionals worldwide for World Glaucoma Week each year in March.

Faces of Glaucoma Campaign (January is Glaucoma Awareness Month)
January is national Glaucoma Awareness Month in the United States.

“Solving Neurodegeneration 2” Catalyst Meeting Convenes Neuroscience Experts in Boston
“Our goal for this meeting was to spark some new ideas and approaches to solving neurodegeneration.”

Elite Paratriathlete Amy Dixon Delivers Keynote at Glaucoma Research Foundation’s 2023 Patient Summit
Visually impaired professional triathlete Amy Dixon was the keynote speaker at our 2023 Patient Summit.

The 2023 Shaffer Grants for Innovative Glaucoma Research
GRF’s $50,000 grants initiate creative research ideas.

Where To Find Credible Information About Glaucoma
Receiving a Glaucoma diagnosis can be scary, especially if you don’t know much about it. Here’s where you can find accurate and credible information.

2023 Shaffer Research Prize Awarded to Dr. Rachel Wang Kuchtey and Dr. Lev Prasov
The Shaffer Prize for Innovative Glaucoma Research recognizes grant recipients whose projects best exemplify the pursuit of breakthrough ideas to better understand and cure glaucoma.