Early Retina Cell Changes in Glaucoma Identified
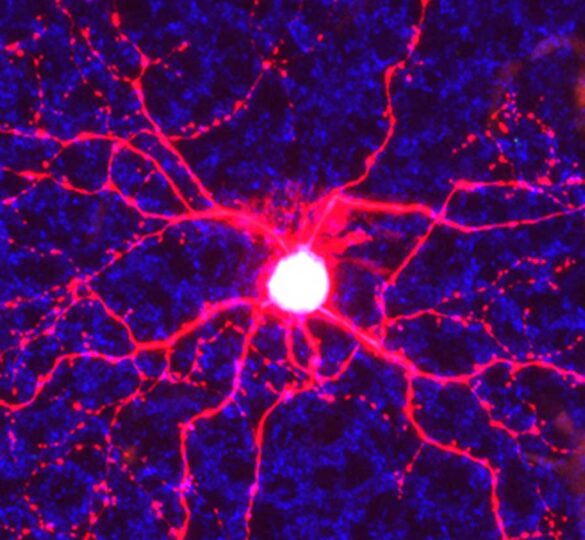
A study published by Andrew D. Huberman, PhD and Rana N. El-Danaf, PhD points to the specific structural features and cell types in the retina that may act as key factors in glaucoma progression.
Glaucoma, the second leading cause of blindness, usually stems from elevated eye pressure, which in turn damages and destroys specialized neurons in the eye known as retinal ganglion cells.
To better understand these cellular changes and how they influence the progression and severity of glaucoma, researchers at University of California, San Diego School of Medicine and Shiley Eye Institute turned to a mouse model of the disease. Their study, published February 10, 2015 in The Journal of Neuroscience, reveals how some types of retinal ganglion cells alter their structures within seven days of elevated eye pressure, while others do not.
“Understanding the timing and pattern of cellular changes leading to retinal ganglion cell death in glaucoma should facilitate the development of tools to detect and slow or stop those cellular changes, and ultimately preserve vision,” said Andrew D. Huberman, PhD, assistant professor of neurosciences, neurobiology and ophthalmology. Huberman co-authored the study with Rana N. El-Danaf, PhD, a postdoctoral researcher in his lab.
Retinal ganglion cells are specialized neurons that send visual information from the eye’s retina to the brain. Increased pressure within the eye can contribute to retinal ganglion cell damage, leading to glaucoma. Even with pressure-lowering drugs, these cells eventually die, leading to vision loss.
In this study, Huberman and El-Danaf used a model engineered to express a green fluorescent protein in specific retinal ganglion cells subtypes. This tool allowed them to examine four subtypes of retinal ganglion cells. The different cell types differ by the location in the eye to which they send the majority of their dendrites (cellular branches). Within seven days of elevated eye pressure, all retinal ganglion cells that send most or all of their dendrites to a region of the eye known as the OFF sublamina underwent significant rearrangements, such as reductions in number and length of dendritic branches. Retinal ganglion cells with connections in the ON part of the retina did not.
“We are very excited about this discovery,” Huberman said. “One of the major challenges to the detection and treatment of glaucoma is that you have to lose a lot of cells or eye pressure has to go way up before you know you have the disease. These results tell us we should design visual field tests that specifically probe the function of certain retinal cells. In collaboration with the other researcher members of the Glaucoma Research Foundation Catalyst for a Cure, we are doing just that and we are confident these results will positively impact human patients in the near-future.” This research was funded, in part, by the Glaucoma Research Foundation Catalyst for a Cure and the E. Matilda Ziegler Foundation for the Blind.
At the time this article was published, Andrew Huberman, PhD was a principal investigator in the Catalyst for a Cure Biomarkers research consortium, a unique approach to collaborative research developed by Glaucoma Research Foundation to accelerate the pace of discovery toward a cure for glaucoma.
Source: University of California San Diego Health Sciences
First posted on February 11, 2015; Last reviewed on May 12, 2022