Catalyst for a Cure 2011 Progress Report
The research team identified a period of vulnerability for retinal ganglion cells early in the disease, when these cells are more sensitive to metabolic insults and stressors.
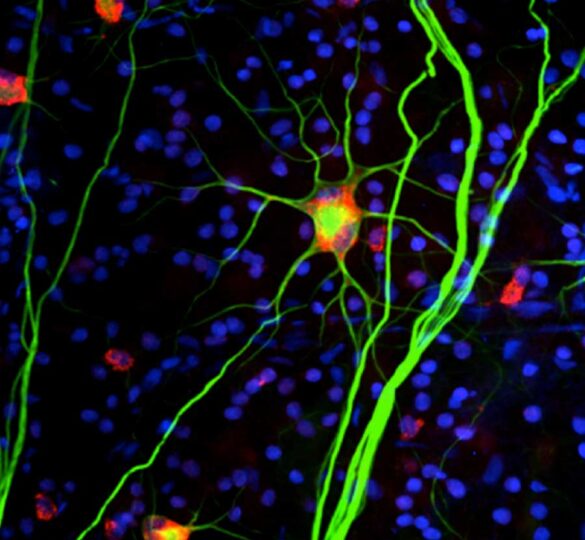
During this past year the investigators of the Catalyst for a Cure (CFC) consortium have continued to probe how and why retinal ganglion cells degenerate in glaucoma. Notably they have investigated how diverse cellular players and molecular pathways contribute to disease onset and progression. Their work will ultimately help define new diagnostic strategies or treatments for glaucoma.
The CFC has delved into the nature of degenerative changes in retinal ganglion cells. They have observed loss of connectivity of these critical neurons in glaucoma, both at the level of inputs with the retina and their outputs to the brain.
These degenerative changes compromise the neuron’s ability to process and transmit visual information well before the neurons actually die. What is becoming clear is that retinal ganglion cells and their axons are challenged very early through factors that the CFC is working to understand.
The team identified a period of vulnerability for retinal ganglion cells early in the disease, when these cells are more sensitive to metabolic insults and stressors. These findings reveal that early changes to retinal ganglion cells may contribute significantly to loss of vision in glaucoma.
Glia Play a Key Role
In addition to these neuronal changes, the CFC researchers are investigating the role of non-neural cells known as glia.
The team previously found that glia become recruited at early stages of the disease, however it was unknown whether this influences the decline of retinal ganglion cells. By blocking the recruitment of glia, the CFC found that retinal ganglion cells as well as visual function can be protected.
These results demonstrate that glia may be detrimental during the initial course of glaucoma, and suggest that dampening the responses of glia may of therapeutic value.
CFC investigators have also established a link between glial activation and oxidative damage in retinal ganglion cells. The team has now established that glia directly regulate retinal ganglion cell homeostasis, and the ability of the neurons to withstand oxidative stress.
Finally, the CFC has discovered a unique population of glia in proximity to retinal ganglion cell axons as they exit the retina. Surprisingly, these glia were found to engulf and degrade materials from intact axons.
The CFC proposes that this newly identified degradation pathway may significantly contribute to the axon loss that defines glaucoma. Together these studies show that the responses of both retinal ganglion cells and surrounding glia are involved in the pathogenesis of glaucoma.
Studies Reveal the Complex Nature of the Glaucoma
The past year has been a productive one for the CFC labs, resulting in 11 publications including papers in the Journal for Neuroscience and the Proceedings of the National Academy of Sciences.
In addition, the CFC made multiple presentations at international meetings, such as the annual meetings of the Society for Neuroscience and the Association for Research in Vision and Ophthalmology.
Together, the studies of the CFC are revealing the complex nature of glaucoma, and how multiple interacting factors ultimately contribute to vision loss in this disease.
Importantly, CFC research has underscored the importance of crosstalk between retinal ganglion cells and surrounding glia in glaucoma progression, suggesting important new strategies for slowing or halting disease.
—
This 2011 progress report is from the Catalyst For a Cure (CFC1) principal investigators:
David J. Calkins, PhD
Vanderbilt Eye Institute
Philip J. Horner, PhD
University of Washington
Nicholas Marsh-Armstrong, PhD
Johns Hopkins School of Medicine, Kennedy Krieger Institute
Monica L. Vetter, PhD
University of Utah
Pictured: Image of retinal ganglion cells from CFC laboratories
First posted onMay 3, 2011; Reviewed on May 20, 2022